EU Commission meeting this week
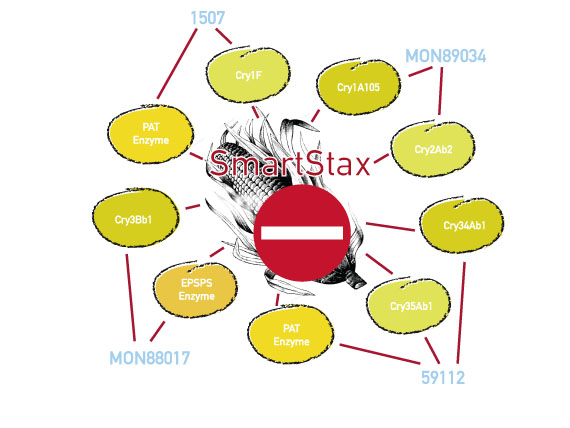 Munich/ Brussels 4 November 2013 Several observers expect the EU Commission to make a decision at a meeting on 6 November on the market authorisation of the genetically engineered maize varieties, SmartStax and Power Core. SmartStax was developed by Monsanto and Dow AgroSciences by crossing several genetically engineered plants. It produces six insecticides and is resistant to two herbicides, glyphosate and glufosinate. This decision has been pending for several months. Meanwhile thousands of concerned European citizens have written to the Commisioner Tonio Borg asking him not to authorise SmartStax for food and feed. In a letter sent to the Commission last week Testbiotech summarised some arguments about why this variety of maize cannot be considered safe. There are a number of substantiated concerns that the genetically engineered plants will have adverse effects on health.
Some of the reasons listed by Testbiotech are:
Munich/ Brussels 4 November 2013 Several observers expect the EU Commission to make a decision at a meeting on 6 November on the market authorisation of the genetically engineered maize varieties, SmartStax and Power Core. SmartStax was developed by Monsanto and Dow AgroSciences by crossing several genetically engineered plants. It produces six insecticides and is resistant to two herbicides, glyphosate and glufosinate. This decision has been pending for several months. Meanwhile thousands of concerned European citizens have written to the Commisioner Tonio Borg asking him not to authorise SmartStax for food and feed. In a letter sent to the Commission last week Testbiotech summarised some arguments about why this variety of maize cannot be considered safe. There are a number of substantiated concerns that the genetically engineered plants will have adverse effects on health.
Some of the reasons listed by Testbiotech are:
- There are indications the maize can trigger immune reactions;
- there are indications that the insecticidal toxin can impact mammalian cells;
- there are new findings showing that humans might take up DNA and other biological active substances from the food to much higher rate than assumed so far;
- no investigations were carried out to examine interactions between the residues from spraying with herbicides and the insecticidal proteins;
- the risk assessment does not comply with necessary scientific standards;
- SmartStax cannot be traced and monitored effectively because there are no adequate methods to identify the maize in food and feed.




